
Phase XXX Type II Diabetes study with renal complications
X October 2017 – Another endocrinology success for MDP Group. MDP Group will partner a European biotech with enrollment open to men and women on a Phase XXX Type II Diabetes study with renal complications. This trial will take place at our sites in Saint Petersburg.

MDP Group is partnering a global CRO on a Phase XXX study in Plaque Psoriasis
X October 2017 – MDP Group is partnering a global CRO on a Phase XXX study in Plaque Psoriasis. The study is open to both women and men from >18 years. Patients will be enrolled at MDP Group sites in Saint Petersburg.

Patients are being recruited into a Phase XXX study in cystitis
X October 2017 – Patients are being recruited into a Phase XXX study in cystitis. Women aged from 18 upwards are being recruited to the trial, which is taking place at MDP Group sites in both Moscow and Saint Petersburg.

MDP Group will begin recruiting patients to new trial
X October 2017 – MDP Group will begin recruiting patients to a Phase XXX clinical trial in mild to moderate bronchial asthma. The study is open to children and adults with a clinical diagnosis of bronchial asthma. This study will take place in Saint Petersburg and Moscow.

MDP has succesfully completed with Joset Asset for 2 weeks
MDP has succesfully completed with Joset Asset for 2 weeks. Test preparation: Joset Asset, tablets, film-coated (INN: Ambroxol, guaifenesin, salbutamol). The comparator: Ascoril®, tablets (INN: Bromhexine, guaifenesin, salbutamol). Inclusion criteria: A signed Information sheet patient consent form for participation in the study. Age from 18 to 65 years inclusive. Men and women. The diagnosis of acute respiratory tract infections, uncomplicated established no earlier than 1...
Read More
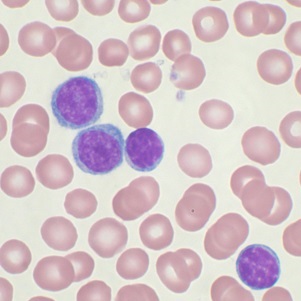
MDP has successfully completed enrolment for study in patients with Chronic lymphocytic leukemia (CLL)
MDP has successfully completed enrolment for study in patients with Chronic lymphocytic leukemia (CLL). It is registration study in Russia Federation. 36 patients with CLL were enrolled during 3 months in 4 sites.

First patient in the phase II Endometrial Carcinoma
MDP CRO enrolled first patient in the phase II Endometrial Carcinoma study at the site of Dr. Gedrevich in Minsk, Belarus
New pharmacy trials
New pharmacy trials

Science is interesting
Science is interesting

Expand your business
Expand your business

