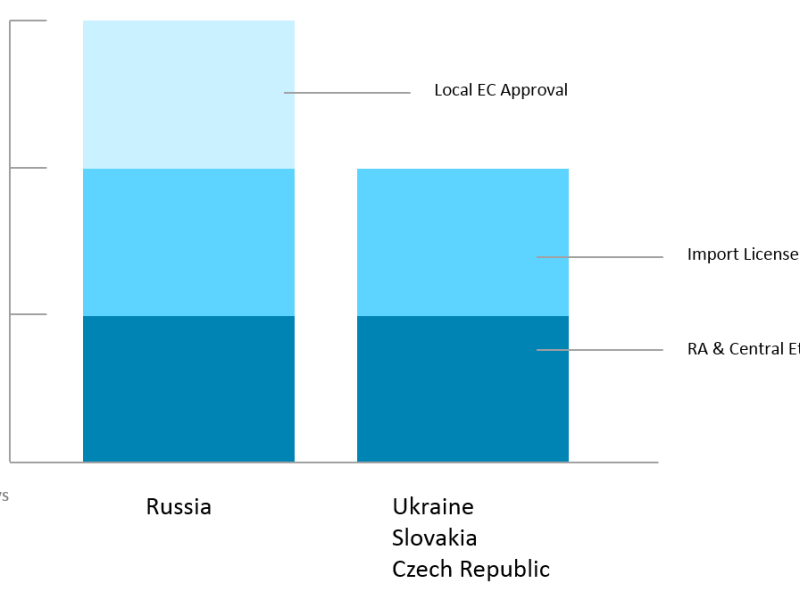Federal Law # 61, 12.04.2010
“On circulation of medicines”
(the latest changes on 12.03.2014)
Legislation for conducting Clinical Trials in Russia
National Standard
GOSTP52379-2005
“Good Clinical
Practice”
The Ministry of Healthcare
Order # 266, 19.06.2003
“Rules for Clinical Practice
in the Russian Federation”
Government
Decrees
etc
…
Primary registration takes 210 days and valid through 5 years.
After 5 years there should be confirmation of the registration that takes 90 days without stopping sales of the drug on the Russian market.
Regulatory timelines in Russia and Easter Europe